Mechanism of a Chemical Reaction
Mechanism of a Chemical Reaction: Overview
This topic covers concepts, such as, Mechanism of a Reaction, Elementary Reactions, Inhibitor & Activation Energy Curve for an Endothermic Reaction etc.
Important Questions on Mechanism of a Chemical Reaction
The minimum energy required to start a chemical reaction is known as:
For a hypothetical reaction , the activation energy for forward and backward reactions are and respectively. The heat of reaction is
The factors which influence the rate of reaction are :
Concentration: Greater the concentrations of the reactants, faster is the rate of reaction.
Temperature: The rate of reaction increases with increase in temperature. For most of the reactions, the rate of reaction becomes almost double with 10o rise in temperature.
Presence of catalyst: A catalyst generally increases the speed of a reaction.
Answer the following question :
The role of a catalyst is to change _____.
Which of the following is NOT the function of catalyst?
A certain physiologically important first-order reaction has activation energy equal to at normal body temperature Without a catalyst, the rate constant for the reaction is . To be effective in the human body, where the reaction is catalysed by an enzyme, the rate constant must be at least If the activation energy is the only factor affected by the presence of the enzyme, by how much must the enzyme lower the activation energy of the reaction to achieve the desired rate? (Report your answer by multiplying with 10 and rounding off to two significant figures)
A certain physiologically important first-order reaction has activation energy equal to at a normal body temperature Without a catalyst, the rate constant for the reaction is . To be effective in the human body, where the reaction is catalysed by an enzyme, the rate constant must be at least . If the activation energy is the only factor affected by the presence of the enzyme, by how much must the enzyme lower the activation energy of the reaction to achieve the desired rate? Give answer to the nearest integer value after multiplying with 10.
After rise in temperature, the activation energy will:
If the activation energy for the forward reaction is and that of the reverse reaction is , what is the enthalpy change for the reaction?
If in a unimolecular reaction, takes place according to the mechanism
I.
II.
where are the rate constants and P, and stand for product molecule, normal molecules of reactants and activated molecules of reactants respectively.
Which of the following statements are correct?
For next two question please follow the same
If in a unimolecular reaction, products takes place according to the mechanism
I.
II.
where are the rate constants and P, and stand for product molecule, normal molecules of reactants and activated molecules of reactants respectively.
Which of the following expressions are correct?
If a reaction is exothermic to the extent of and the forward reaction has an activation energy , the activation energy for the reverse reaction is
In the presence of a catalyst, the heat evolved or absorbed during the reaction ______.
The role of a catalyst is to change _____.
The reaction between and occurs in the following steps:
The reaction intermediate in the reaction is
The energy of activation for reaction is . What will be the activation energy for
Identify the correct potential energy vs reaction co-ordinate graph, which is consistent with given mechanism.
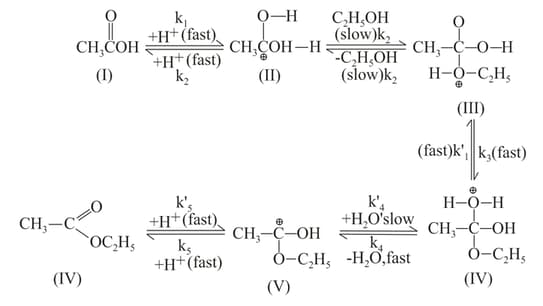
The mechanism of esterification in presence of acid catalyst (H2SO4) is proposed as follows:
Which of the following is definitely true, if for a reaction activation energies of forward and backward reactions are equal?
For a reaction, which of the following reaction properties changes, if the reaction is done in presence of a catalyst?
What is the order of catalytic efficiency of P, Q, R representing the relative case of a homogeneous catalytic reaction that can take place through three alternative paths as depicted below?
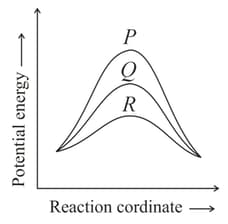
From the above information given, what will be the threshold energy of the catalysed reaction, if the normal energy of the reactant is .
